Isabel, Y10, explores the comprehensibility of Dmitri Mendeleev’s traditional periodic table and whether it would be more accessible for younger children and enhance learning methods if it were flipped around by 180˚.
The Periodic Table is an important symbol in Chemistry and since Dmitri Mendeleev’s discovery of the Periodic system in 1869, it has remained the same for 150 years; but could turning it 180˚ make important concepts easier to understand, especially in teaching younger children?
This year has been announced the Year of (Mendeleev’s) Periodic Table which has become the generic way of arranging the elements. However, some scientists like Martyn Poliakoff and his team have started to question the comprehensibility of it. After extensive research, they decided to flip the traditional arrangement upside down, so that the information is more understandable and intuitively ordered.
The research team argues that this presentation is more helpful and has many benefits. Firstly, when the table is flipped the properties of the elements such as atomic mass and proton number now increase from bottom to top therefore making more numerical sense. Secondly, it represents the Aufbau principal more accurately, which states that electrons fill up ‘shells’ from low to high energy. Finally, when young children are trying to learn from the table, the more relevant elements to them are located towards the bottom of the table, making its use quicker and more accessible. Therefore, in lessons, students will not have to look all the way to the top of the table to be able to find the right information.
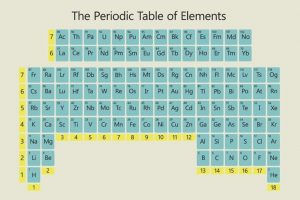
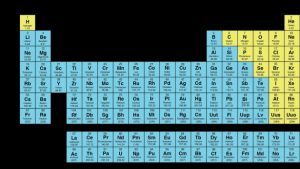
However, when I compared the two versions of the periodic table myself, I found that the traditional form of the table made more sense to me for many reasons. For example, in both situations I found my eyes drawn to the top row of elements, so it did not matter that the elements that I use the most were on the bottom row. However, this could be put down to a force of habit, so I also asked my 10-year-old brother to look at the two perspectives of the table and see where he looked. He immediately pointed to the top of both and when I asked him the reason he said that from top to bottom ‘is the way you read’ so the properties make more sense going down from top to bottom. He also seemed to prefer the traditional table, commenting that it was like a ‘pyramid’ in the way the numbers were arranged and was a much clearer way to display the elements.
Whilst some may argue that the arrangement of the table is more effective if it were upside down, for me the traditional version of the periodic table works just as well. Testing this principle to a larger group will allow different models to be tried to see if it makes understanding the periodic table easier for younger learners.
References:
Martyn Poliakoff et al, Nat. Chem., 2019, https://www.nature.com/articles/s41557-019-0253-6




 lp to discover how many parents and pupils you have in your class with scientific interests and skills.
lp to discover how many parents and pupils you have in your class with scientific interests and skills.

 Imagine if we could replay, in slow motion, our favourite demos, to watch the magic of reality unfold frame by frame. Imagine always being able to see the demonstration clearly, regardless of where you were in the class. Imagine if we had a backup in case a demonstration, for whatever reason, went awry. Imagine if we were teaching a different topic entirely, and felt that now would be a wonderful time to illustrate our point with a display, but there was no time to throw it together. (Imagine if you wanted to show all your friends really cool science videos…)
Imagine if we could replay, in slow motion, our favourite demos, to watch the magic of reality unfold frame by frame. Imagine always being able to see the demonstration clearly, regardless of where you were in the class. Imagine if we had a backup in case a demonstration, for whatever reason, went awry. Imagine if we were teaching a different topic entirely, and felt that now would be a wonderful time to illustrate our point with a display, but there was no time to throw it together. (Imagine if you wanted to show all your friends really cool science videos…)
 “Woah!”
“Woah!” “WOAAAAH!”
“WOAAAAH!”